- +91 9321330888
- info@crystapeaksolutions.com
- Sr.No. 201, Hiss.No. 1159, SADESATRA NALI, HADAPSAR, PUNE 411028 MAHARASHTRA
X-Ray Fluorescence (XRF) is a powerful quantitative and qualitative analytical tool to determine the elemental composition of material.
The analysis determines the types of elements in the sample based on the characteristic wavelengths of the X-rays emitted by the atoms.
XRF is capable of detecting elements from Boron (B) to Uranium (U) from ppm range to 100%.
X-Ray fluorescence is used in a wide range of applications, including
Cement production
Soil surveys
Mining (e.g., for measurement the grade of ore)
Ceramic and Glass manufacturing Industries
Petroleum industry (e.g., sulfur content of crude oils and petroleum products)
Field analysis in geological and environmental studies
A prepared sample specimen is excited by the radiation of an X-Ray. This causes an electron from the inner electron shells to get knocked-off. Electrons from outer electron shells drop-in to fill the resultant voids emitting a fluorescence radiation characteristic in its energy distribution for a particular element/material. This fluorescence radiation is measured by the detector. The energies or wavelengths of the emitted x-rays are used to identify the elements present in the sample while the concentrations of the elements are determined by the intensity of the x-rays.
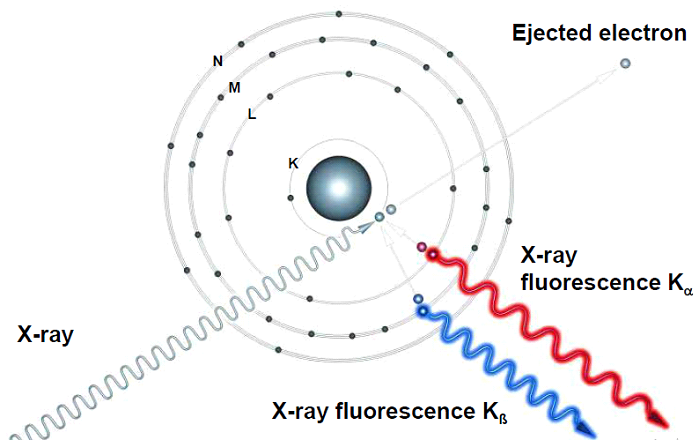
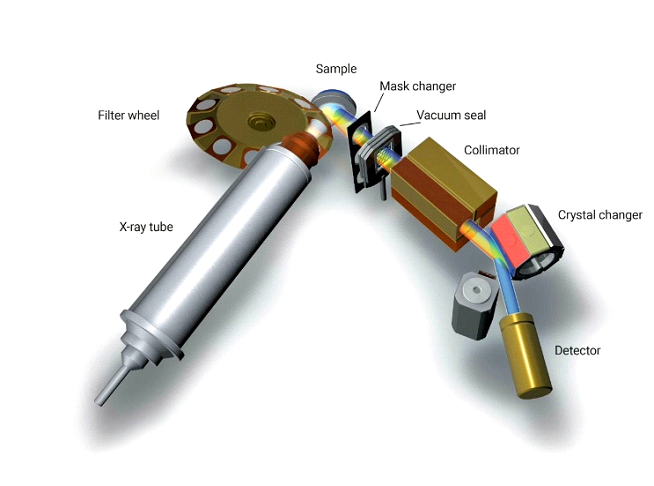
Image Courtesy by Bruker AXS GmbH Germany
Solid samples can be anything from pieces of metal (polished metal
samples) or electronics or plastics piece cut properly. The ideal sample for XRF analysis will have a
perfectly flat surface.
Rough surfaces can cause scattering.
Pressed Pellets
A fine powder mixing it with a binding /grinding aid
and then pressing the mixture in a die at between 20 and 30T to produce a homogeneous sample pellet.
Ideally to a grain size of <75um


